Milpro Film-Coated Allwormer Tablets for Cats (2-8kg)
Product Information
Milpro Wormer Tablets offer effective treatment and control of all major intestinal worms in cats and dogs, including roundworm, hookworm, whipworm, tapeworm, and hydatids. When used monthly, they also prevent heartworm.
Key Features:
- Broad-Spectrum Efficacy: Treats and controls a wide range of intestinal worms.
Heartworm Prevention: Monthly use helps prevent heartworm.
- Highly Palatable: Innovative liver-flavoured film-coating ensures easy administration.
- Convenient Dosage: Small, ovoid tablets are easy to hide in food or administer by hand.
- Tailored Dosing: Available in various sizes with scored tablets for accurate dosing.
Milpro is the trusted choice for a safer, more compliant worming regimen.
Recommended For:
Cats and kittens from 6 weeks of age and weighing at least 0.5kg
The dog formulation is not suitable for use in cats.
Composition
Small Cats & Kittens
- 10 mg Praziquantel
- 4 mg Milbemycin Oxime
Cats
- 40 mg Praziquantel
- 16 mg Milbemycin Oxime
Indications
For Cats:
Treats and controls roundworm (Toxocara cati, Toxascaris leonina), hookworm (Ancylostoma tubaeforme), tapeworm (Dipylidium caninum, Taenia spp.) and prevents heartworm (Dirofilaria immitis).
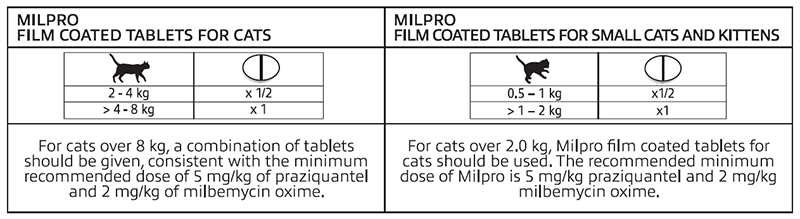
- Adult cats should be treated every 3 months unless lifestyle factors indicate a tailored treatment protocol.
- Pregnant queens should be treated at mating, before birth of kittens and then every 3 months.
- Nursing queens should be treated at the same time as their kittens.
Dosage and Direction for Use
- Animals should be weighed to ensure accurate dosing.
- Milpro Tablets should be administered orally, either with or after food.
- Half tablets may be kept for use in the original blister for up to six months after the first half was used.
- When administering Milpro Tablets, be certain that the entire dose is consumed. Watch for several minutes following treatment to be sure that the entire dose has been eaten. lf the entire dose is not eaten, redose as soon as possible, with the full dose.
- Fleas are intermediate hosts for tapeworm (Dipylidium caninum). Effective flea control will reduce the incidence of tapeworm.
- If worm problems persist, consult a veterinarian.
Contraindications
Precautions
- Caution should be taken in the case of concurrent use of the product and other macrocyclic lactones.
- Studies with milbemycin oxime indicate that the margin of safety in certain dogs of Collie or related breeds is less than in other breeds. In these dogs, the recommended dose should be strictly observed. Clinical signs in Collies are similar to those seen in the general dog population when overdosed.
- Milpro given monthly, may be used for prevention of heartworm infection of dogs.
- In heartworm risk areas, or in the case of unknown heartworm status, an examination for existing heartworm infection prior to commencement of treatment is recommended. The use in dogs suffering from microfilaremia is not recommended. Consult a veterinarian.
- The safety of Milpro in heartworm positive cats has not been established. An examination for existing heartworm infection prior to commencement of treatment is recommended. Consult a veterinarian.
Shipping
Non-Prescription Products:
• Free shipping on metro orders over $49+.
• Shipping fee for regional orders & metro orders under $49 will be calculated at checkout.
Prescription Medications:
Shipped separately via express shipping at a flat rate of $8.95.
Returns
Non-Prescription Products:
We offer a hassle-free return policy within 30 days for unopened items in original condition for exchange or store credit. Refunds are available if the product is faulty or not as described; however, food or treats cannot be returned once opened.
Prescription Medications:
We cannot accept change of mind returns for prescription products, given that there are laws that prohibit the resale of medication. Please make your selections carefully, and double-check your order before submitting.
Palatability Guarantee
Our Palatability Guarantee applies to selected brands, including Advance, Royal Canin, Purina, Hill's and 4CYTE, allowing you to return any unused portion of dry/wet food for an exchange or full refund.
Frequently Asked Questions
MILPRO delivers broad spectrum protection against common intestinal worms and when given every 3 months treats and controls hookworm, roundworm and tapeworm in cats. One tablet per pack.
Milpro is a generic hybrid and as such the trials carried out for the registration dossier are assessed for ethical purposes and only essential and critical studies are permitted. As milbemycin and praziquantel has been previously studied in the reference product for use in pregnancy a trial for Milpro was not performed. Therefore the SPC states - use during pregnancy and lactation only according to a benefit / risk assessment by the responsible veterinarian.






































